At BookChem, we specialize in surfactant science that powers personal care, home care, and industrial formulations. Surfactants are essential ingredients in many daily-use products, functioning as cleansing agents, emulsifiers, solubilizers, and foaming agents. Understanding the types and working principles of surfactants is critical for effective formulation development.
What Are Surfactants?
Surfactants, or surface-active agents, are compounds that lower the surface tension between two substances—commonly between a liquid and a solid or between two liquids. Their unique structure consists of:
- Hydrophilic Head (water-loving)
- Hydrophobic Tail (oil-loving)
This dual-affinity allows surfactants to interact with both water and oils, making them highly effective in emulsifying, cleaning, dispersing, and foaming applications.
Major Types of Surfactants
Surfactants are typically classified based on the charge present on the hydrophilic head group:
1. Anionic Surfactants
- Charge: Negative
- Function: Strong detergency, excellent foaming
- Examples: Sodium Lauryl Sulfate (SLS), Sodium Laureth Sulfate (SLES), Alpha Olefin Sulfonate (AOS)
- Applications: Shampoos, body washes, laundry detergents
Anionic surfactants are highly effective at removing dirt and oils, which makes them a staple in cleansing products. They are often combined with other surfactants to improve mildness and stability.
2. Cationic Surfactants
- Charge: Positive
- Function: Conditioning, antimicrobial, fabric softening
- Examples: Quaternary Ammonium Compounds (e.g., CTAC, BTAC)
- Applications: Hair conditioners, fabric softeners, antiseptic formulations
Cationic surfactants are attracted to negatively charged surfaces like hair or textiles, making them ideal for smoothing and antistatic properties. They are not typically used with anionic surfactants due to charge incompatibility.
3. Nonionic Surfactants
- Charge: None
- Function: Emulsification, solubilization, mild cleansing
- Examples: Alkyl Polyglucosides (APGs), Cocamide DEA, PEG derivatives
- Applications: Baby care, facial cleansers, industrial emulsions
Nonionic surfactants are known for their mildness and high compatibility with other surfactant types. They perform well under varying pH and water hardness conditions.
4. Amphoteric (Zwitterionic) Surfactants
- Charge: Dual (positive and negative depending on pH)
- Function: Mildness enhancer, foam booster, thickener
- Examples: Cocamidopropyl Betaine, Lauryl Hydroxysultaine
- Applications: Sulfate-free shampoos, body cleansers, baby products
Amphoteric surfactants offer excellent skin compatibility and are often used to reduce the irritation potential of anionic surfactants in sensitive skin products.
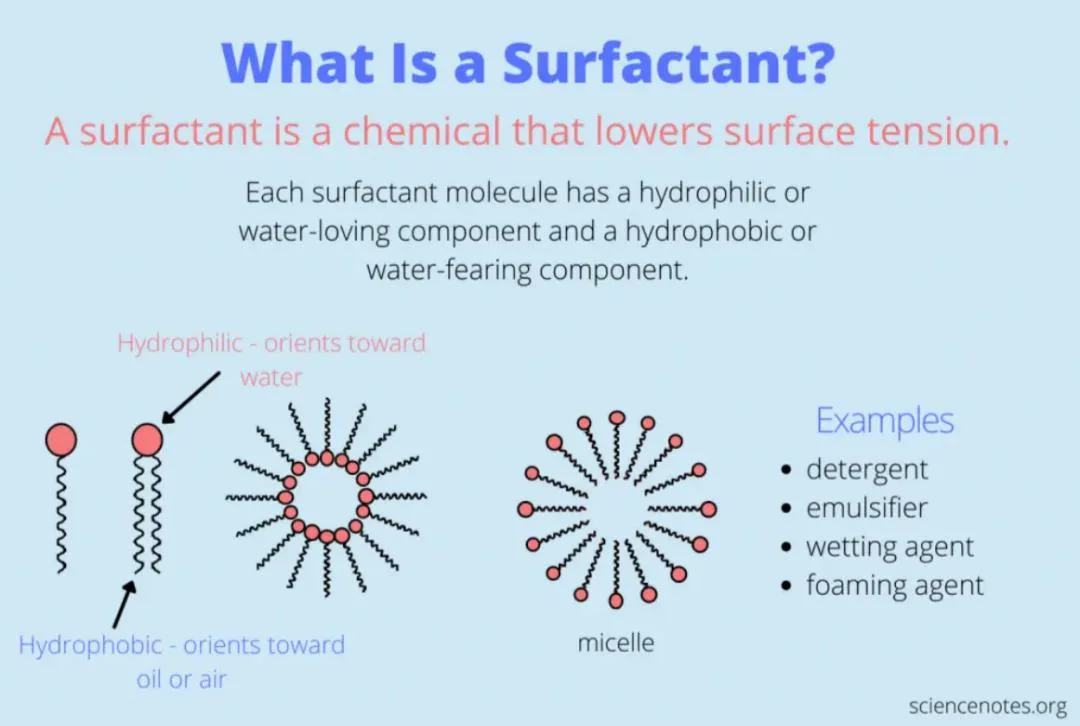
Surfactant Mechanism: How They Work
Surfactants work by aligning themselves at the interface between water and oil (or dirt). When used in a cleaning product:
- The hydrophobic tail embeds itself into oily soils.
- The hydrophilic head remains in the water phase.
- Agitation (rubbing or shaking) causes micelle formation, where oil and dirt are trapped inside spherical structures and rinsed away with water.
This mechanism is also fundamental to emulsification, wetting, dispersion, and foaming effects.
Choosing the Right Surfactant with BookChem
At BookChem, we offer a wide portfolio of surfactants to suit diverse formulation needs across industries:
- Personal Care: Mild cleansing, conditioning, foaming
- Home Care: Strong detergency, stability under hard water
- Industrial Applications: Solubilizing oils, dispersing solids, emulsifying chemicals
Our R&D experts provide tailored guidance based on your product’s function, target market, and regulatory requirements.
Ready to Formulate Smarter?
Whether you’re creating sulfate-free skincare or high-foaming detergents, BookChem has the surfactant solution to match.
Get in Touch with Us to explore samples, specifications, and application support.
